Effectiveness of Ivermectin as add-on Therapy in COVID-19 Management (Pilot Trial)
Faiq I Gorial, Sabeeh Mashhadani, Hend M Sayaly, Basim Dhawi Dakhil, Marwan M Almashhadani, Adnan M Aljabory, Hassan M Abbas, Mohammed Ghanim, Jawad I Rasheed
doi:10.1101/2020.07.07.20145979
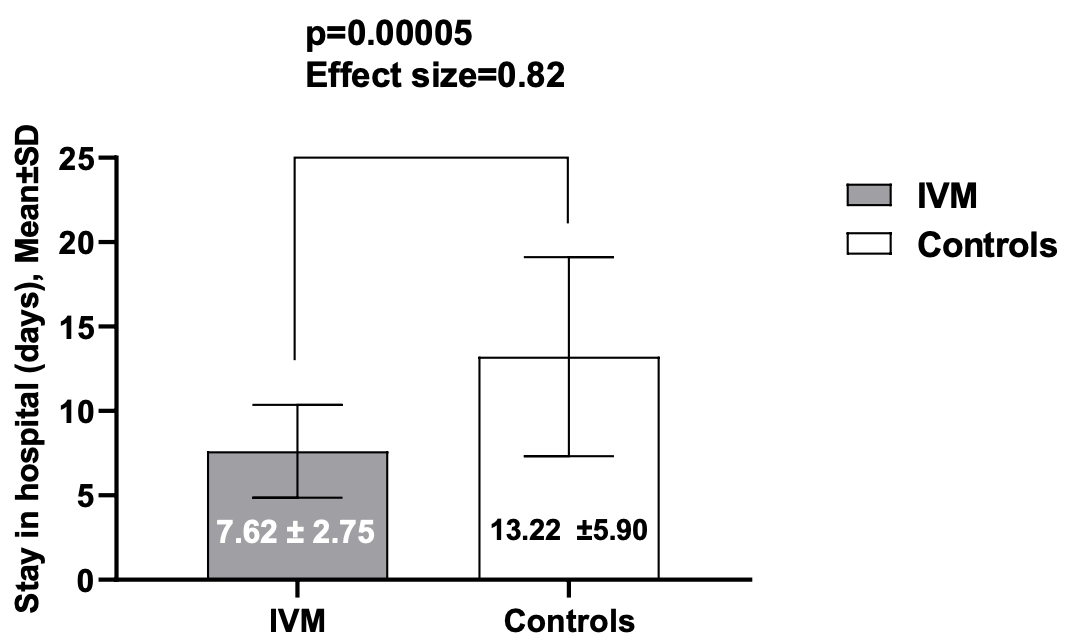
Background: To date no effective therapy has been demonstrated for COVID-19. In vitro, studies indicated that ivermectin (IVM) has antiviral effect. Objectives: To assess the effectiveness of ivermectin (IVM) as add-on therapy to hydroxychloroquine (HCQ) and azithromycin (AZT) in treatment of COVID-19. Methods: This Pilot clinical trial conducted on hospitalized adult patients with mild to moderate COVID-19 diagnosed according to WHO interim guidance. Sixteen Patients received a single dose of IVM 200Mcg /kg on admission day as add on therapy to hydroxychloroquine ( HCQ)and Azithromycin (AZT) and were compared with 71 controls received HCQ and AZT matched in age, gender, clinical features, and comorbidities. The primary outcome was percentage of cured patients, defined as symptoms free to be discharged from the hospital and 2 consecutive negative PCR test from nasopharyngeal swabs at least 24 hours apart. The secondary outcomes were time to cure in both groups and evaluated by measuring time from admission of the patient to the hospital till discharge. Results: Of 87 patients included in the study,t he mean age ± SD (range) of patients in the IVM group was similar to controls [44.87 ± 10.64 (28-60) vs 45.23 ± 18.47 (8-80) years, p=0.78] Majority of patients in both groups were male but statistically not significant [11(69%) versus 52 (73%), with male: female ratio 2.21 versus 2.7-, p=0.72) All the patients of IVM group were cured compared with the controls [ 16 (100 %) vs 69 (97.2 %)]. Two patients died in the controls. The mean time to stay in the hospital was significantly lower in IVM group compared with the controls (7.62 ± 2.75 versus 13.22 ±5.90 days, p=0.00005, effect size= 0.82). No adverse events were observed Conclusions : Add-on use of IVM to HCQ and AZT had better effectiveness, shorter hospital stay, and relatively safe compared with controls. however, a larger prospective study with longer follow up may be needed to validate these results.
References
Borba, Val, Sampaio, Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial, JAMA Netw Open
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Research Available online
Canga, Prieto, Liébana, Martínez, Vega et al., The pharmacokinetics and interactions of ivermectin in humans-a mini-review, The AAPS journal
Cao, Wang, Wen, A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe COVID-19, N Engl J Med,
doi:10.1056/NEJMoa2001282Cao, Wang, Wen, A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19, N Engl J Med
Chen, Liu, Lui, A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 COVID-19), J Zhejiang Univ Sci
Choudhary, Sharma, Potential use of hydroxychloroquine, ivermectin and azithromycin drugs in fighting COVID-19: trends, scope and relevance, New Microbes and New Infections
Gautret, Hydroxychloroquine and azithromycin as a treatment of COVID19: results of an open-label non-randomized clinical trial, Int J Antimicrob Agents,
doi:10.1016/j.ijantimicag.2020.105949Gautret, Lagier, Parola, Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study, Travel Med Infect Dis,
doi:10.1016/j.tmaid.2020.101663Gautret, Lagier, Parola, Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial
Grein, Ohmagari, Shin, Compassionate Use of Remdesivir for Patients with Severe COVID-19
Helmy, Fawzy, Elaswad, Sobieh, Kenney et al., The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control, J Clin Med
Liu, Cao, Xu, Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell Discov
Molina, Delaugerre, Goff, No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection, Med Mal Infect,
doi:10.1016/j.medmal.2020.03.006Patel, Usefulness of Ivermectin in COVID-19 Illness
Poschet, Perkett, Timmins, Deretic, Azithromycin and ciprofloxacin have a chloroquine-like effect on respiratory epithelial cells, bioRxiv,
doi:10.1101/2020.03.29.008631Whitehead, Julious, Cooper, Campbell, Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for acontinuous outcome variable, Stat Meth Med Res
Yao, Ye, Zhang, In vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
DOI record:
{
"DOI": "10.1101/2020.07.07.20145979",
"URL": "http://dx.doi.org/10.1101/2020.07.07.20145979",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>To date no effective therapy has been demonstrated for COVID-19. In vitro, studies indicated that ivermectin (IVM) has antiviral effect.</jats:p></jats:sec><jats:sec><jats:title>Objectives</jats:title><jats:p>To assess the effectiveness of ivermectin (IVM) as add-on therapy to hydroxychloroquine (HCQ) and azithromycin (AZT) in treatment of COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>This Pilot clinical trial conducted on hospitalized adult patients with mild to moderate COVID-19 diagnosed according to WHO interim guidance. Sixteen Patients received a single dose of IVM 200Mcg /kg on admission day as add on therapy to hydroxychloroquine (HCQ)and Azithromycin (AZT) and were compared with 71 controls received HCQ and AZT matched in age, gender, clinical features, and comorbidities.</jats:p><jats:p>The primary outcome was percentage of cured patients, defined as symptoms free to be discharged from the hospital and 2 consecutive negative PCR test from nasopharyngeal swabs at least 24 hours apart. The secondary outcomes were time to cure in both groups and evaluated by measuring time from admission of the patient to the hospital till discharge.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Of 87 patients included in the study,t he mean age ± SD (range) of patients in the IVM group was similar to controls [44.87 ± 10.64 (28-60) vs 45.23 ± 18.47 (8-80) years, p=0.78] Majority of patients in both groups were male but statistically not significant [11(69%) versus 52 (73%), with male: female ratio 2.21 versus 2.7-, p=0.72)</jats:p><jats:p>All the patients of IVM group were cured compared with the controls [16 (100 %) vs 69 (97.2 %)]. Two patients died in the controls. The mean time to stay in the hospital was significantly lower in IVM group compared with the controls (7.62 ± 2.75 versus 13.22 ±5.90 days, p=0.00005, effect size= 0.82). No adverse events were observed</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Add-on use of IVM to HCQ and AZT had better effectiveness, shorter hospital stay, and relatively safe compared with controls. however, a larger prospective study with longer follow up may be needed to validate these results.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2020,
7,
8
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2760-5566",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gorial",
"given": "Faiq I",
"sequence": "first"
},
{
"affiliation": [],
"family": "Mashhadani",
"given": "Sabeeh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sayaly",
"given": "Hend M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dakhil",
"given": "Basim Dhawi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "AlMashhadani",
"given": "Marwan M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aljabory",
"given": "Adnan M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abbas",
"given": "Hassan M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ghanim",
"given": "Mohammed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rasheed",
"given": "Jawad I",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
7,
8
]
],
"date-time": "2020-07-08T20:25:15Z",
"timestamp": 1594239915000
},
"deposited": {
"date-parts": [
[
2021,
1,
15
]
],
"date-time": "2021-01-15T01:01:54Z",
"timestamp": 1610672514000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2024,
3,
28
]
],
"date-time": "2024-03-28T12:37:58Z",
"timestamp": 1711629478616
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 34,
"issued": {
"date-parts": [
[
2020,
7,
8
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2020.07.07.20145979",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2020,
7,
8
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2020,
7,
8
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.3390/jcm9041225",
"article-title": "The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control",
"doi-asserted-by": "crossref",
"first-page": "E1225",
"issue": "4",
"journal-title": "J Clin Med",
"key": "2021011308350749000_2020.07.07.20145979v1.1",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19",
"doi-asserted-by": "crossref",
"first-page": "1787",
"journal-title": "N Engl J Med",
"key": "2021011308350749000_2020.07.07.20145979v1.2",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.8857",
"article-title": "Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial",
"doi-asserted-by": "crossref",
"first-page": "e208857",
"issue": "4",
"journal-title": "JAMA Netw Open",
"key": "2021011308350749000_2020.07.07.20145979v1.3",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1038/s41421-020-0156-0",
"article-title": "Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro",
"doi-asserted-by": "crossref",
"first-page": "16",
"journal-title": "Cell Discov",
"key": "2021011308350749000_2020.07.07.20145979v1.4",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa237",
"doi-asserted-by": "publisher",
"key": "2021011308350749000_2020.07.07.20145979v1.5"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105949",
"doi-asserted-by": "publisher",
"key": "2021011308350749000_2020.07.07.20145979v1.6"
},
{
"DOI": "10.1101/2020.03.29.008631",
"doi-asserted-by": "crossref",
"key": "2021011308350749000_2020.07.07.20145979v1.7",
"unstructured": "Poschet JF , Perkett EA , Timmins GS , Vojo Deretic . Azithromycin and ciprofloxacin have a chloroquine-like effect on respiratory epithelial cells. bioRxiv 2020.03.29.008631; doi: https://doi.org/10.1101/2020.03.29.008631"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"doi-asserted-by": "crossref",
"key": "2021011308350749000_2020.07.07.20145979v1.8",
"unstructured": "Caly L , Druce JD , Catton MG , Jans DA , Wagstaff KM . The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Research Available online 3 April 2020, 104787"
},
{
"DOI": "10.1208/s12248-007-9000-9",
"article-title": "The pharmacokinetics and interactions of ivermectin in humans—a mini-review",
"doi-asserted-by": "crossref",
"first-page": "42",
"issue": "1",
"journal-title": "The AAPS journal",
"key": "2021011308350749000_2020.07.07.20145979v1.9",
"volume": "10",
"year": "2008"
},
{
"DOI": "10.1177/0962280215588241",
"doi-asserted-by": "publisher",
"key": "2021011308350749000_2020.07.07.20145979v1.10"
},
{
"key": "2021011308350749000_2020.07.07.20145979v1.11",
"unstructured": "U.S Food and Drug Administration. Real World Evidence Program. 2018;(February)."
},
{
"DOI": "10.1016/j.tmaid.2020.101663",
"doi-asserted-by": "publisher",
"key": "2021011308350749000_2020.07.07.20145979v1.12"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105949",
"doi-asserted-by": "publisher",
"key": "2021011308350749000_2020.07.07.20145979v1.13"
},
{
"key": "2021011308350749000_2020.07.07.20145979v1.14",
"unstructured": "Patel A. Usefulness of Ivermectin in COVID-19 Illness. Available at SSRN 3580524. 2020 Apr 19."
},
{
"DOI": "10.1016/j.medmal.2020.03.006",
"doi-asserted-by": "publisher",
"key": "2021011308350749000_2020.07.07.20145979v1.15"
},
{
"DOI": "10.1056/NEJMoa2001282",
"doi-asserted-by": "publisher",
"key": "2021011308350749000_2020.07.07.20145979v1.16"
},
{
"DOI": "10.1056/NEJMoa2007016",
"doi-asserted-by": "publisher",
"key": "2021011308350749000_2020.07.07.20145979v1.17"
},
{
"article-title": "A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 COVID-19)",
"first-page": "215",
"issue": "2",
"journal-title": "J Zhejiang Univ Sci",
"key": "2021011308350749000_2020.07.07.20145979v1.18",
"volume": "49",
"year": "2020"
},
{
"DOI": "10.1016/j.nmni.2020.100684",
"doi-asserted-by": "crossref",
"key": "2021011308350749000_2020.07.07.20145979v1.19",
"unstructured": "Choudhary R , Sharma AK . Potential use of hydroxychloroquine, ivermectin and azithromycin drugs in fighting COVID-19: trends, scope and relevance. New Microbes and New Infections. 2020 Apr 22:100684."
}
],
"reference-count": 19,
"references-count": 19,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2020.07.07.20145979"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Effectiveness of Ivermectin as add-on Therapy in COVID-19 Management (Pilot Trial)",
"type": "posted-content"
}